Moderna starts testing coronavirus vaccine on kids aged 12 to 17 despite dangers of negative side effects
12/29/2020 / By Zoey Sky
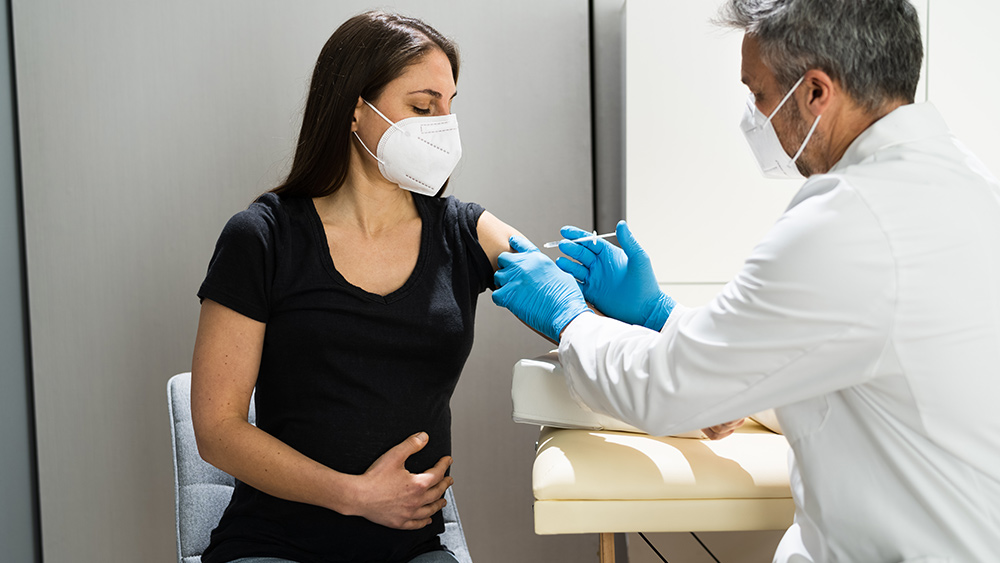
The coronavirus (COVID-19) pandemic continues to ravage countries across the world, with desperate people counting on a vaccine that can help protect them from this infectious disease. And yet despite warnings from health experts about potential negative side effects, Moderna Inc. has already begun testing its experimental coronavirus vaccine on kids aged 12 to 17.
Moderna’s vaccine to be completed by 2022
According to a press release from the biotechnology company, the phase II/III trial will involve an estimated 3,000 volunteers aged 12 to 17.
The researchers will give half of the volunteers two shots of Moderna’s immunization or mRNA-1273 four weeks apart. Meanwhile, the rest of the participants will receive saltwater placebos.
The scientists claim that they will monitor the safety and efficacy of the vaccine in children. All volunteers will be followed for 12 months after the second inoculation.
In a statement, Moderna CEO Stephane Bancel announced that the company is “pleased to begin this Phase 2/3 study of mRNA-1273 in healthy adolescents in the US.” Bancel added that the company aims to generate data by spring in 2021 that will “support the use of mRNA-1273 in adolescents in advance of the 2021 school year.”
Bancel concluded that Moderna Inc. wants to produce a “safe vaccine” that can help protect adolescents and ensure that they can soon “return to school in a normal setting.”
Clinicaltrials.gov, a registry of clinical trials run by the US National Library of Medicine, revealed that the Moderna Inc. study is slated to be completed by June 2022. The company’s vaccine will also be used at testing locations in Idaho, Minnesota, New York, Oklahoma, Texas and Utah.
When asked, a Moderna spokeswoman didn’t disclose how many children have been enrolled as of writing.
Other companies have already been testing vaccines on children
Pfizer Inc., another vaccine manufacturer, has already been testing its coronavirus vaccine in children aged 12 since October. Another company, AstraZeneca, has also started testing young participants outside America.
But the testing seems unwarranted, especially since children rarely develop severe coronavirus symptoms. Additionally, young patients seldom require hospitalization for COVID-19, highlighting the danger of testing vaccines on children, who haven’t been previously tested in trials for experimental shots. (Related: French infectious disease expert warns about dangers of COVID-19 vaccine.)
Last October, the Centers for Disease Control and Prevention (CDC) released a statement announcing that clinical trials only included healthy, non-pregnant adults, at least until this December. The CDC added that the recommended groups might in the future as clinical trials expand to recruit more people volunteers.
How does Moderna’s vaccine work?
Moderna’s vaccine was developed in collaboration with the National Institutes of Health.
The vaccine uses part of the pathogen’s genetic code called messenger RNA (mRNA) to help a patient’s body recognize COVID-19 and attack if they get infected. The vaccine candidate then tricks the body into producing some of the viral proteins, which the immune system recognizes and builds a defensive response.
Back in November, clinical trial data revealed that the vaccine is 94.1 percent effective at preventing COVID-19. The Moderna vaccine is also allegedly 100 percent effective at preventing severe disease.
On Dec. 17., the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee voted in favor of the FDA granting an emergency use authorization (EUA) to its mRNA-based coronavirus vaccine candidate, mRNA-1273. From the panel, 20 members voted in favor of providing the EUA. None of the members voted against it.
Previously, the same committee voted 17-4 recommending Pfizer and BioNTech’s mRNA-based vaccine, BNT162b2. One member abstained during both advisory committee meetings.
The recommendation was based on study findings presented by Moderna, along with data from the pivotal phase III COVE study that evaluated mRNA-1273 in adult patients. The potential vaccine had an efficacy rate of 94.1 percent during primary efficacy analysis and it offered full protection against severe COVID-19 cases.
According to a press release, most of the negative side effects caused by the Moderna vaccine occurred within the first one to two days after injection and lasted for one to two days.
Moderna is ready to ship 20 million doses to the U.S. government by the end of 2020 after the potential EUA. The company has signed an agreement to deliver an additional 180 million doses of mRNA-1273 to the government by the first half of 2021.
The U.S. government can also opt to purchase an additional 300 million doses of mRNA-1273. Moderna has reported that it can deliver one billion doses worldwide by 2021. The company is also working on the efficient distribution of the vaccine.
Moderna’s potential vaccine can remain stable at standard refrigerator temperatures of 2 to 8 C for 30 days. The company can also cover local transport and that it can distribute the vaccine under controlled conditions in a liquid state at the standard temperature.
Pfizer and BioNTech have also received EUA for their vaccine in at least six countries such as America and the United Kingdom. But conditional marketing authorization requests for both candidates remain under review in Europe.
On the other hand, AstraZeneca and Oxford University’s potential vaccine candidate AZD1222 was, on average, at least 70 percent effective in preventing coronavirus based on interim data from late-stage studies.
J&J and CureVac’s COVID-19 vaccine candidates are also in late-stage development, with study data slated for a 2021 release date.
The side effects of Moderna’s vaccine
Children are often the last group tested during clinical trials since their bodies and immune systems behave differently, and this means they might have different treatment needs.
Children may also require different doses or needle sizes depending on their height, weight and age. This is one reason why most children are only vaccinated after safety has been well-documented in the adult population.
Several volunteers have reported the following symptoms after taking the Moderna vaccine:
- Chills
- Fevers
- Headache
- Pain in the arm
- Shortness of breath
Last September, Luke Hutchison, a 44-year-old volunteer in Moderna’s phase 3 trial, reported that he’d had a mild fever after taking the first shot of the mRNA-1273 vaccine.
He also experienced “full-on COVID-like symptoms” after getting the second shot. Hutchison also experienced side effects like cough, muscle and joint aches, really hot hands and feet and general malaise.
Jack Morningstar, another volunteer, said that he experienced fatigue after the first dose and fever after the second. Morningstar, a college student from the University of North Carolina at Chapel Hill, said that since the trial was double-blind, he has no way of confirming if he received the real vaccine or the placebo until the vaccine cleared FDA authorization.
Two other volunteers in Moderna’s study experienced similar side effects, but they declined to go on the record. Hutchinson shared that he wanted to go public about his experience because he was worried that Moderna might keep people in the dark about the vaccine’s possible negative side effects.
Are you willing to subject your children to the adverse effects of Moderna’s vaccine?
Visit Pandemic.news for more updates on coronavirus vaccines and their side effects.
Sources include:
Submit a correction >>
Tagged Under:
Big Pharma, Child abuse, children's health, coronavirus, covid-19, COVID-19 vaccine, guinea pigs, Moderna, mRNA, pandemic, vaccine trials, vaccine wars, vaccines
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
VaccineDamage.News is a fact-based public education website published by Vaccine Damage News Features, LLC.
All content copyright © 2018 by Vaccine Damage News Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.